UNODC: CND decision on international control of eutylone and three precursors enters into force in November 2022
VIENNA, Austria – November 2022: The decision adopted by the Commission on Narcotic Drugs (CND) during its 65th Session from 14 to 18 March 2022 to add eutylone to Schedule II of the Convention on Psychotropic Substances of 1971, was communicated by the Secretary-General on 27th of May 2022 to all States Members of the United Nations, to non-member States Parties to the Conventions, to the World Health Organization and to the International Narcotics Control Board and entered into force on 23 November 2022. In addition, the decision of the CND to add the precursors norfentanyl, N-phenyl-4-piperidinamine (4-AP) and tert-butyl 4-(phenylamino)piperidine-1-carboxylate (1-boc-4-AP) to Table I of the Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 also entered into force on 23 November 2022.
Eutylone is a synthetic cathinone, with structural and pharmacological similarities to 3,4-methylenedioxymethamphetamine (MDMA), methylone, and pentylone. Seizure reports indicate that eutylone is mostly distributed as tablets, capsules, crystals, and pills, suggesting oral administration as the primary route of administration. (WHO 2021) No therapeutic use for eutylone is known and deaths had been reported as a result of eutylone use. Reported severe adverse events in these cases included hyperthermia, hypertension and seizures (CND 2022).
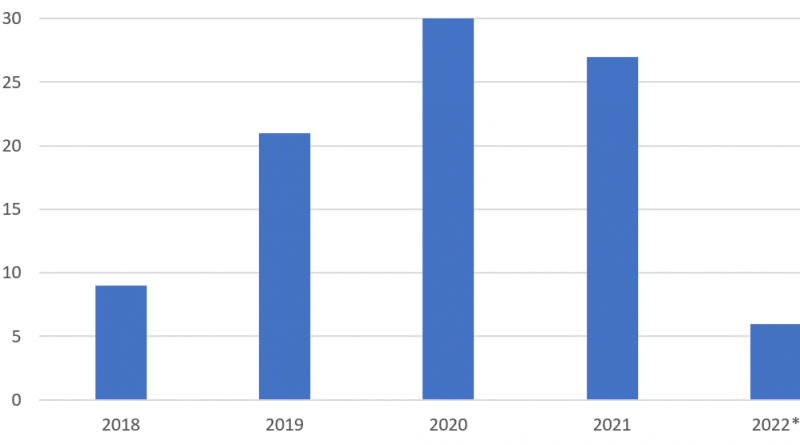
Eutylone was reported to the UNODC EWA for the first time in 2013. Within the last five years, the substance was reported by an increasing number of countries and territories to the UNODC EWA with a peak in 2020 with 30 reporter countries and territories (see Figure).
Figure: Eutylone identifications in seized material reported to the UNODC EWA 2018-2022*

Source: UNODC Early Warning Advisory on NPS, November 2022. *Note: Data for 2022 include information made available to UNODC until October 2022.
Eutylone has further been reported in post-mortem and clinical admission cases submitted to the UNDOC EWA in 2020 and 2021 by countries in East and South-East Asia, North America and Oceania.

For further information please see:
UNODC EWA newsclip: June 2022 – UNODC: CND decision on international control of brorphine and metonitazene enters into force – remaining decisions will enter into force in November 2022
UNODC EWA newsclip: March 2022 – UNODC: Three NPS “scheduled” at the 65th Session of the Commission on Narcotic Drugs
WHO, Critical Review Report: Eutylone (September 2021).
CND, Report on the sixty-fifth session (United Nations, 2022).
UNODC Toxicology Portal of the Early Warning Advisory on NPS